Abstract
Introduction: Hemophilia A is a rare bleeding disorder caused by pathogenic variants in the F8 gene, resulting in insufficient factor VIII (FVIII) activity. Adeno-associated virus (AAV)-mediated gene transfer enables the delivery of a modified functional F8 coding sequence to hepatocytes that subsequently synthesize FVIII at levels that may prevent bleeding events in the absence of exogenous FVIII. We present updated results with nearly 2-year follow-up from the Alta study (NCT03061201), an ongoing gene therapy study in patients with severe hemophilia A (FVIII activity <1%).
Methods: The phase 1/2 Alta study is a dose-ranging study of giroctocogene fitelparvovec (PF-07055480 and previously called SB-525), a recombinant AAV serotype 6 vector encoding a modified B-domain-deleted F8 coding sequence. Giroctocogene fitelparvovec was infused into adults aged ≥18 years with severe hemophilia A in 4 cohorts of 2 patients each across 4 ascending doses: 9e11, 2e12, 1e13, and 3e13 vg/kg. The 3e13-vg/kg dose cohort was expanded with 3 additional patients. Key end points included safety, circulating FVIII activity, use of FVIII replacement therapy, and frequency of bleeding events. We present data with nearly 2 years of follow-up from the ongoing Alta study (NCT#03061201; data cutoff date: May 19, 2021).
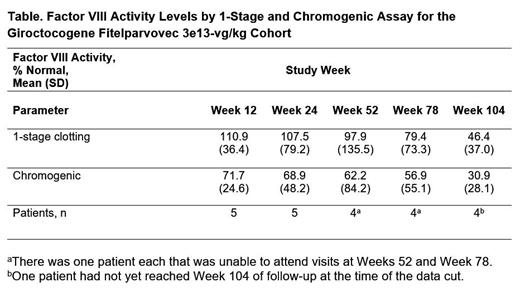
Results: Eleven male patients participated in the study (mean [SD] age, 30.3 [7.8] years; white, 81.8%). As of the cutoff date, one patient had not completed 2 years (104 weeks) of follow-up resulting in patients having been followed for 95 to 195 weeks overall. The most commonly reported treatment-related adverse events (AEs; n/N [%]), included elevated liver enzymes and infusion-related reactions: increased alanine aminotransferase (ALT; 5/11 [45.5%] overall; 3/5 [60.0%] in the 3e13-vg/kg cohort), increased aspartate aminotransferase (AST; 3/11 [27.3%] overall; 2/5 [40.0%] in the 3e13-vg/kg cohort), pyrexia (3/11 [27.3%] overall; 3/5 [60.0%] in the 3e13-vg/kg cohort), and tachycardia (2/11 [18.2%] overall; 2/5 [40.0%] in the 3e13-vg/kg cohort). Treatment-related serious AEs were reported in 1 patient (in the 3e13-vg/kg cohort) who experienced hypotension and fever with onset ≈6 hours after giroctocogene fitelparvovec infusion; the events fully resolved with treatment and did not delay post-infusion discharge the next day. ALT elevations requiring >7 days of corticosteroid treatment were observed in 4 of the 5 patients in the 3e13-vg/kg cohort: elevations in ALT were managed with a tapering course of corticosteroids (median 58 days; range: 11-134 days), with maintenance of efficacious levels of FVIII activity, as evidenced by a lack of bleeding events around the time of corticosteroid treatment and minimal bleeding events afterwards. No patient in the study developed an inhibitor to FVIII, and there have been no thrombotic events and no hepatic masses detected. Patients in the 3e13-vg/kg cohort had mean FVIII activity maintained in the mild to normal range through week 104 for the 4 patients in this cohort with available data at this time point (Table). In this cohort, the annualized bleeding rate [(number of all bleeding episodes starting 3 weeks after study drug infusion)/(observation period in years)] was 0 for the first year postinfusion and 0.9 throughout the total duration of follow-up. In the 3e13-vg/kg cohort, 2 patients experienced a total of 3 bleeding events (2 traumatic; 1 unknown) necessitating treatment with exogenous FVIII; 1 of these events occurred in a target joint. No patients in cohort 4 have resumed prophylaxis.
Conclusions: A single infusion of giroctocogene fitelparvovec gene therapy in patients with severe hemophilia A was generally well tolerated with associated increases in FVIII levels in the mild to normal range, without sustained AEs, and with minimal bleeding in the highest-dose cohort (3e13 vg/kg). A phase 3 study (NCT04370054) of giroctocogene fitelparvovec in patients with hemophilia A is ongoing.
Visweshwar: Biogen Idec: Membership on an entity's Board of Directors or advisory committees. Leavitt: Pfizer: Research Funding; Rigel: Consultancy; HEMA Biologics: Consultancy; BPL: Consultancy; Behring: Consultancy; Syntimmune: Research Funding; Sangamo Therapeutics: Research Funding; BioMarin: Consultancy, Research Funding; Catalys: Consultancy; CSL DOVA: Consultancy; Merck: Consultancy. Konkle: Genentech USA Inc.: Honoraria; Sigilon Therapeutics: Honoraria; BioMarin Pharmaceutical Inc.: Other: Data and safety monitoring; CSL Behring: Other: Data and safety monitoring. Giermasz: ATHN: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; NovoNordisk: Consultancy; UniQure: Consultancy, Research Funding; Sanofi Genzyme: Consultancy; Bioverativ/Sanofi: Consultancy, Research Funding, Speakers Bureau; Sangamo Therapeutics,: Research Funding; BioMarin: Consultancy, Research Funding. Stine: Applied Stem Cell Therapeutic: Consultancy; BioMarin: Consultancy. Rupon: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Di Russo: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Tseng: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. de los Angeles Resa: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Ganne: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Agathon: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Plonski: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Rouy: Sangamo Therapeutics: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Ended employment in the past 24 months. Cockroft: Sangamo Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Fang: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Arkin: Pfizer Inc.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal